BUSINESS OPPORTUNITY
Leading scientists at the Oslo University Hospital have identified a unique biomarker in patients with basal-like breast cancer, which indicates a favorable prognosis. This group of patients largely represents those with triple-negative breast cancer.
Currently, the majority of these patients undergo chemotherapy before or after surgery. This treatment can lead to significant and long-lasting side effects. With the discovery of this biomarker, there’s potential to determine which patients might not require such intensive treatment, thereby avoiding its associated complications. The identified CTLA4 biomarker has a great potential for being implemented in gene expression tests commercially available.
Inven2 AS seeks partners for licensing of the technology.
TECHNOLOGY
An actionable immune-related biomarker identifying basal-like breast cancer patients with an excellent prognosis has been identified and the clinical utility of the biomarker has been demonstrated on three independent cohorts (Oslo1, METABRIC and SCAN-B).
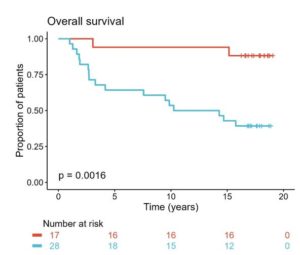
Figure 1 | Treatment outcomes by CTLA4 expression in Oslo1 cohort. Kaplan-Meier plots of outcomes in patients with tumor CTLA4 expression above and below the cutoff at the 63rd percentile.
ADVANTAGE
The research team is developing a diagnostic test based on this biomarker’s expression. The test could help clinicians discern which patients have a favorable prognosis and might be candidates for reduced chemotherapy regimens. Still, the scientists emphasize the need to ensure that such treatment modifications don’t compromise the patients’ survival outcomes.
IPR
A patent application protecting the biomarker is filed.

