Background
Colorectal cancer (CRC) is the third most common cancer worldwide. Despite fair survival for localized CRC, metastatic CRC has a five-year survival rate of only ~11%. Neoantigens are excellent targets for adoptive cellular immunotherapy since they are exclusively expressed on tumor cells and are not subject to central tolerance in the host immune cells. Neoantigens are considered to make colon cancer with high microsatellite instability (MSI-H) more immunogenic than CRC with low MSI (MSI-L). Although immunotherapy with immune checkpoint inhibitors can provide good antitumor effects, 30-50% of MSI-H CRC patients are unresponsive to immune checkpoint inhibitors. Radium-1 is the first mRNA TCR T cell therapy targeting a neoantigen tested in clinic for MSI-H CRC. It demonstrates the feasibility of this approach in advanced solid tumor.
Business Opportunity
The safety and feasibility of Radium-1 in a patient with solid malignancy have been reported. Due to its excellent safety profile, these findings warrant further investigation in a Phase I/II study. Inven2 seeks collaborators and licensors that can support the continued clinical development of Radium-1.
Technology
The neoantigen-specific TCR, Radium-1, was isolated from a patient with long term clinical response to peptide vaccination in a Phase I clinical trial. In pre-clinical studies, the Radium-1 TCR redirected T cells against CRC cell lines expressing the targeted TGFβRII frameshift mutation which is seen in 90% of MSI-H CRC. Furthermore, T cells transduced with the Radium-1 TCR significantly reduced the growth of CRC and enhanced survival in a xenograft NOD/SCID mouse model.
Recently, a first-in-human study of Radium-1 TCR in a patient with advanced metastatic CRC was completed (NCT03431311). mRNA electroporation for transient TCR expression was used to reduce the risk of off-tumor toxicity. The results from this first patient demonstrate that the therapy is feasible and safe. The treatment was well tolerated with stabilization of tumor size and the patient surpassed our conceived life expectancy.
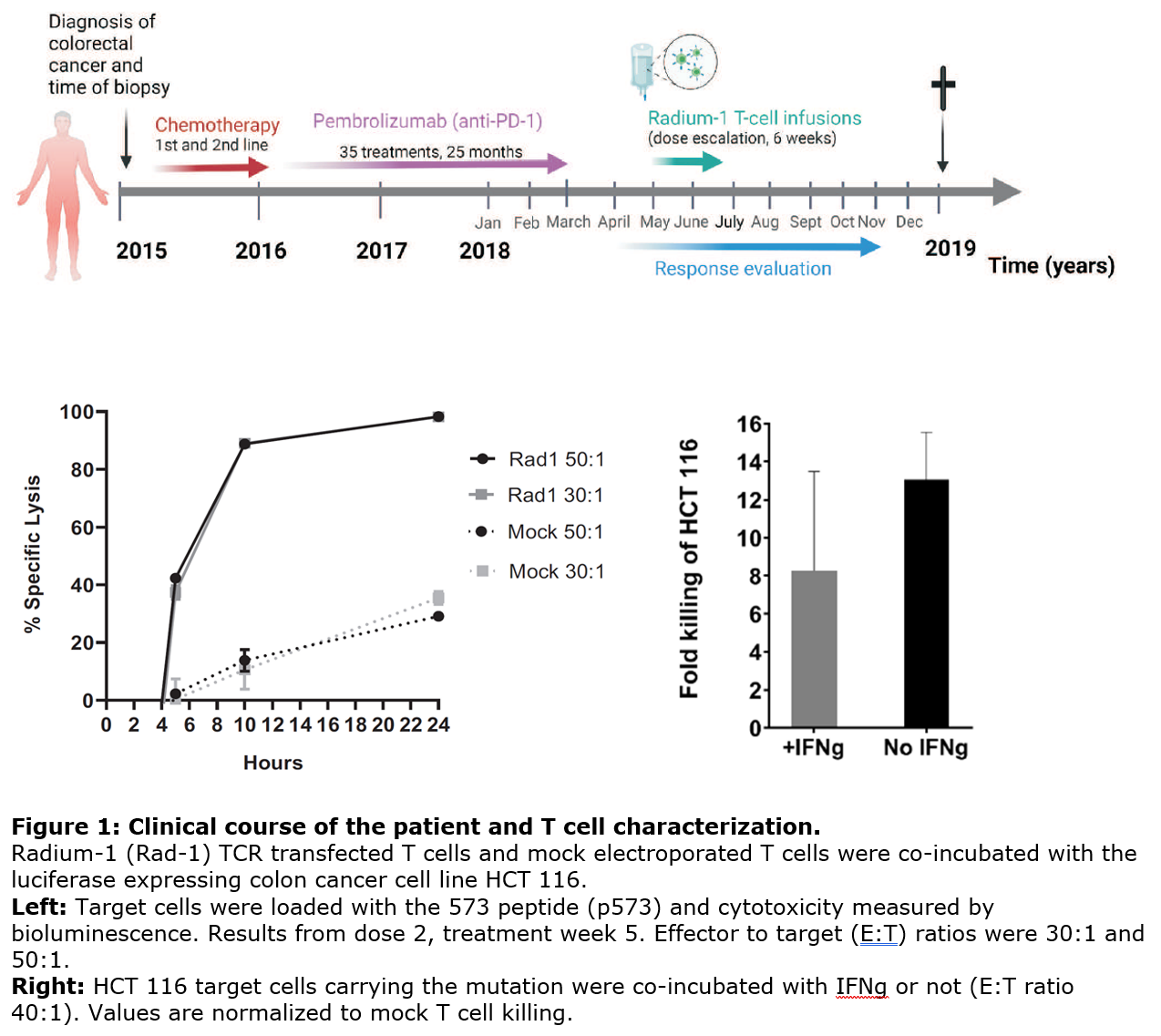
Intellectual Property
A patent is granted in EPO and China.
References
- Inderberg, Else M., et al. “T cell therapy targeting a public neoantigen in microsatellite instable colon cancer reduces in vivo tumor growth.” Oncoimmunology4 (2017): e1302631.
- Mensali, Nadia, et al. “Preclinical assessment of transiently TCR redirected T cells for solid tumour immunotherapy.” Cancer Immunology, Immunotherapy68 (2019): 1235-1243.
- Maggadottir et al. “Transient TCR-based T-cell therapy in a patient with advanced treatment-resistant MSI-high colorectal cancer” (in revision)


