Pharmacy agreement
When a hospital pharmacy shall deliver services in a clinical study, a separate agreement must be entered into between the hospital pharmacy and the Sponsor/CRO.
One of the following agreement templates must be used for this agreement:
- Master Pharmacy Agreement; to enter into a master-agreement for several clinical studies with a hospital pharmacies enterprise.
- Pharmacy Agreement ; to enter into an agreement with hospital pharmacy for a specific clinical study.
-
Pharmacy Agreement Destruction; to enter into an agreement with hospital pharmacy for destruction of study drug only
Inven2 is responsible for negotiating the legal part of the pharmacy agreement on behalf of the four hospital pharmacy enterprises in Norway, which own in total 31 hospital pharmacies.
The hospital pharmacy is responsible for specifying the tasks of the pharmacy and the price of these services.
The agreement and budget process with hospital pharmacy per study:
- Sponsor/CRO must provide information in the notification form on whether a hospital pharmacy shall deliver services, including being responsible for import of study medication.
- Based on the information, Inven2 will initiate the process for the pharmacy agreement and will inform Sponsor/CRO about the further process:
- Sponsor/CRO must enter study and site details in the agreement template and send the document to Inven2
- Sponsor/CRO must clarify which services are requested and the prices for these directly with the contact person at the hospital pharmacy
- One hospital pharmacy within each hospital pharmacy enterprise is responsible for clarifying which services are needed and the prices. The agreed services and prices will apply to all hospital pharmacies owned by the same hospital pharmacy enterprise
- When the agreement (legal) text is verified by Inven2, and the services including prices are agreed upon between Sponsor/CRO and hospital pharmacy, the agreement can be signed. The execution of the agreement is finalized directly between each hospital pharmacy and Sponsor/CRO, without the involvement of Inven2.
For more information:
Hospital Pharmacy Enterprise South-Eastern Norway (Sykehusapotekene HF): sykehusapotekene.no/fag-og-forskning/forskning/kliniske-studier
Hospital Pharmacy Enterprise Western Norway (Sjukehusapoteka Vest HF): https://sjukehusapoteka-vest.no/
Hospital Pharmacy Enterprise Central Norway (Sykehusapotekene i Midt-Norge HF): https://sykehusapoteket.no/
Hospital Pharmacy Enterprise Northern Norway (Sykehusapotek Nord HF): https://sykehusapotek-nord.no/
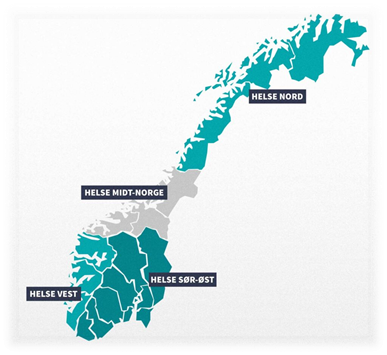
Organization of hospital pharmacies in Norway:
The hospital pharmacies enterprises are separate entities from the hospital enterprises, and the four regional hospital pharmacies enterprises in Norway own in total 34 hospital pharmacies. See detailed overview below:

Do you want to know more?

Erlend Carlson Cand.Pharm.
Clinical Contract Manager
Clinical & Industry Collaboration
Tlf. 97 70 07 17
