Background
Ovarian cancer is the leading cause of death from gynaecological malignancies in the Western world. CAR-T cell therapy has been tested against various targets in ovarian cancer and one of the candidates is the MUC16 ectodomain. This ectodomain remains on the cell surface after cleavage of cancer-antigen 125 (CA125), the domain distal from the membrane, which is currently used as a serum biomarker for ovarian cancer. Scientists at Oslo University hospital have demonstrated the suitability of the CA125 as a target for CAR-T cell therapy.
Business Opportunity
In vitro and in vivo results, including PDX studies, demonstrate that the CA125 domain of MUC16 represents an excellent target for treating MUC16-positive malignancies. Inven2 seeks collaboration with industrial partners and licensors who can support the clinical development of K101 CAR T cell therapy in patients with ovarian cancer.
Technology
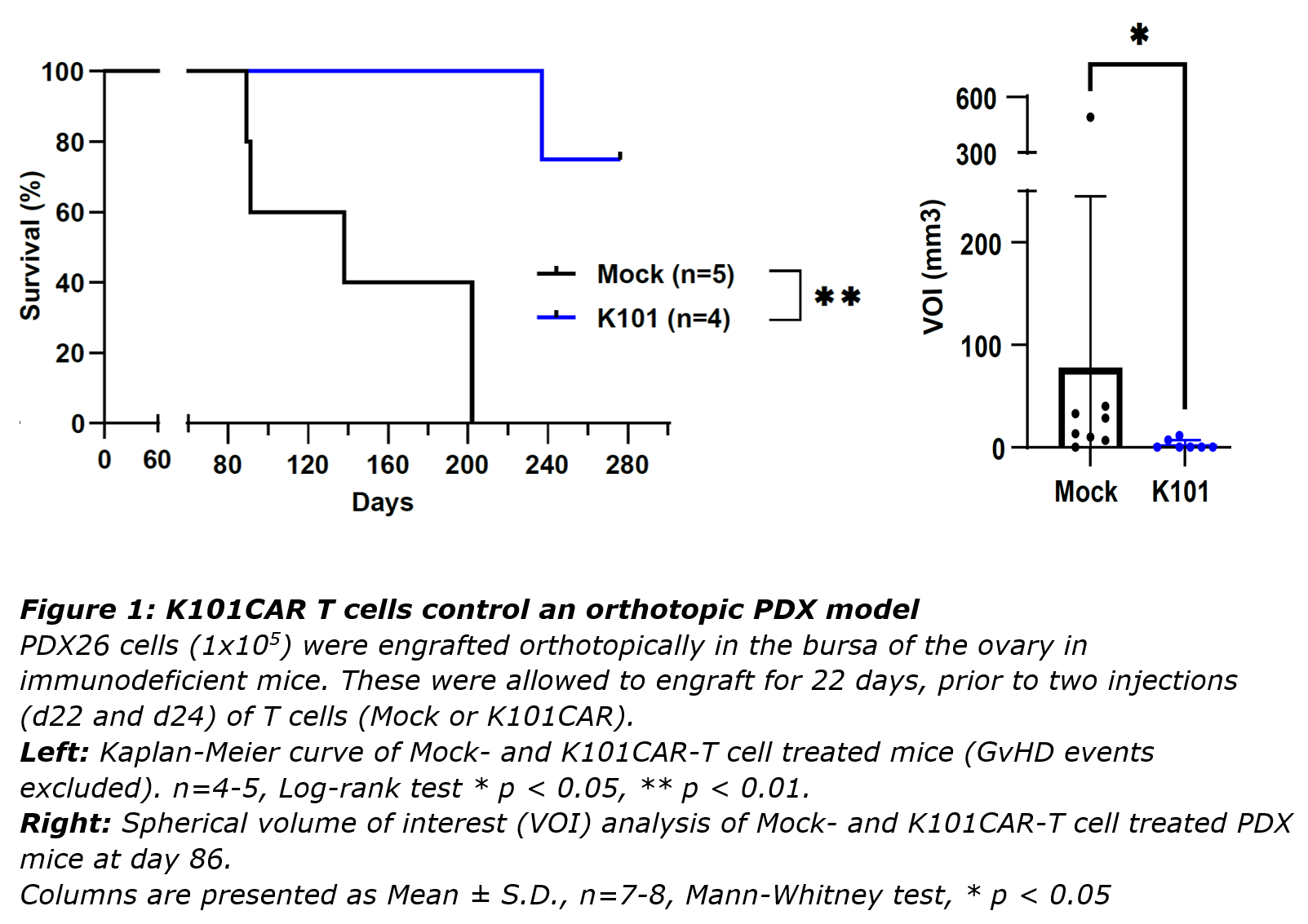
A series of antibodies raised against the CA125 extracellular repeat domain of MUC16 were tested and adapted to CAR format. Comparisons between these candidates, and against an existing CAR targeting the MUC16 ectodomain, identified K101 as having high potency and specificity.
A high efficacy of K101CAR T cells was observed against cell lines and patient-derived tumours, in vitro and in vivo. It was also demonstrated that K101CAR functionality was not impaired by the soluble antigen. In direct comparisons, K101CAR, which targets the extracellular repeat domains, was shown to have comparable efficacy to the previously validated 4H11CAR, which targets the MUC16 ectodomain.
Intellectual Property
A patent application has been filed.
References


