Clinical studies
Clinical trials are the key to medical development. On average, patients who participate in clinical trials get access to experimental treatment several years before the treatment becomes available on the market.
Inven2 is responsible for managing the agreement and all financial aspects of clinical studies. We focus on efficient and quick agreement processes, in order to make our hospitals become attractive partners for the industry. In 2021, Inven2 managed 417 ongoing clinical studies and 177 new studies were registered in that year.
Managing 20 clinical trials at the same time
The Clinical Trial Unit is located next to the cardiac monitoring unit and in the heart of Rikshospitalet University Hospital, both in a literal and in a figurative sense. We are known for our high-quality deliveries in demanding early-phase trials’, says Kristin Sandnes, clinical trial coordinator.
Information
WHY CONDUCT CLINICAL TRIALS IN NORWAY?
Inven2 is responsible for the agreement and financial aspects of clinical trials and industry cooperation. We work actively to attract more international and Norwegian companies to conduct their trials in Norway. We work closely with hospitals, companies, the ecosystem and the authorities to encourage this. In this article, you can read more about what makes Norway an attractive country in which to conduct trials.
Latest clinical trial news
More news
Latest clinical trial news
These are news regarding Inven2’s work within clinical trials, articles about ongoing and new studies, and other relevante clinical trials news.
Oslo University Hospital Selected for Groundbreaking Clinical Study
The Clinical Research Unit at Oslo University Hospital was selected as the sole centre worldwide to conduct a "first-in-human" study of an entirely...
Summary of the year 2024
2024 ended on a positive note. Zelluna and Ultimovacs merged in an exciting collaboration to advance their precision cell therapies into clinical...
Summary of the year 2023
Inven2 is grateful to everyone who has contributed to building business and helping patients through new inventions, clarification, clinical...
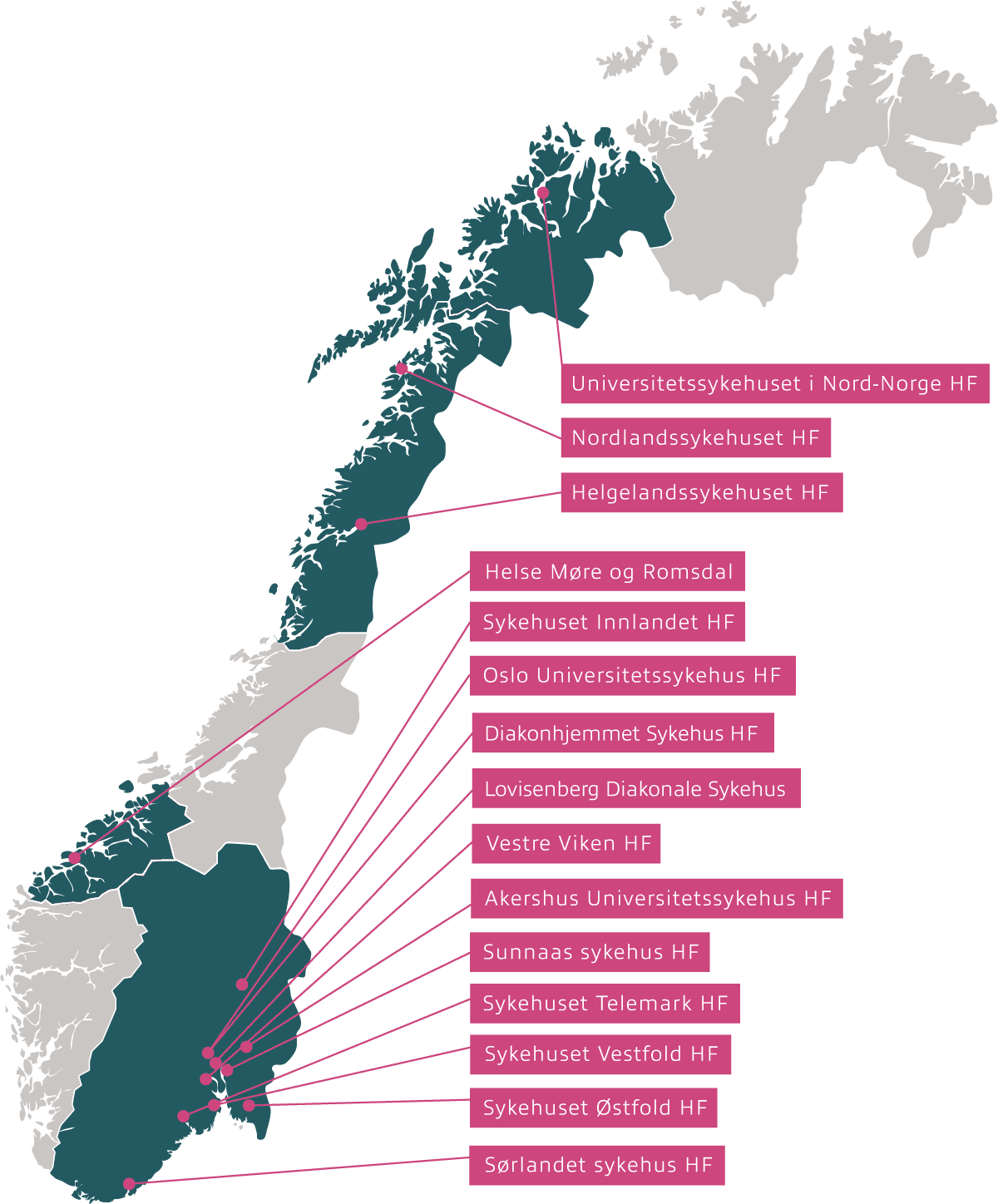
Inven2 is responsible for the following hospitals:
- Akershus University Hospital Trust
- Oslo University Hospital Trust
- Diakonhjemmet Hospital
- Lovisenberg Diaconal Hospital
- Nordland Hospital Trust
- Helgeland Hospital Trust
- Sunnaas Hospital Trust
- Vestfold Hospital Trust
- Innlandet Hospital Trust
- Telemark Hospital Trust
- Hospital of Southern Norway Trust
- Østfold Hospital Trust
- Vestre Viken Hospital Trust
- University Hospital of North Norway
- Hospital pharmacy enterprise
- Sjukehusapoteka Vest HF
- Sykehusapotekene i Midt-Norge HF
- Sykehusapotek Nord HF
- Helse Møre og Romsdal Hospital Trust
Ongoing studies
On the website helsenorge.no you can find a regularly updated overview of ongoing clinical studies across Norway.



