Inven2’s role and responsibilities
Inven2 is responsible for managing the agreement and financial aspects of clinical trials and industry cooperation that is funded in whole or in part by industry.
Inven2 is responsible for contract negotiation and financial follow-up for clinical studies on behalf of 17 Hospital Trusts (HF) in Norway:
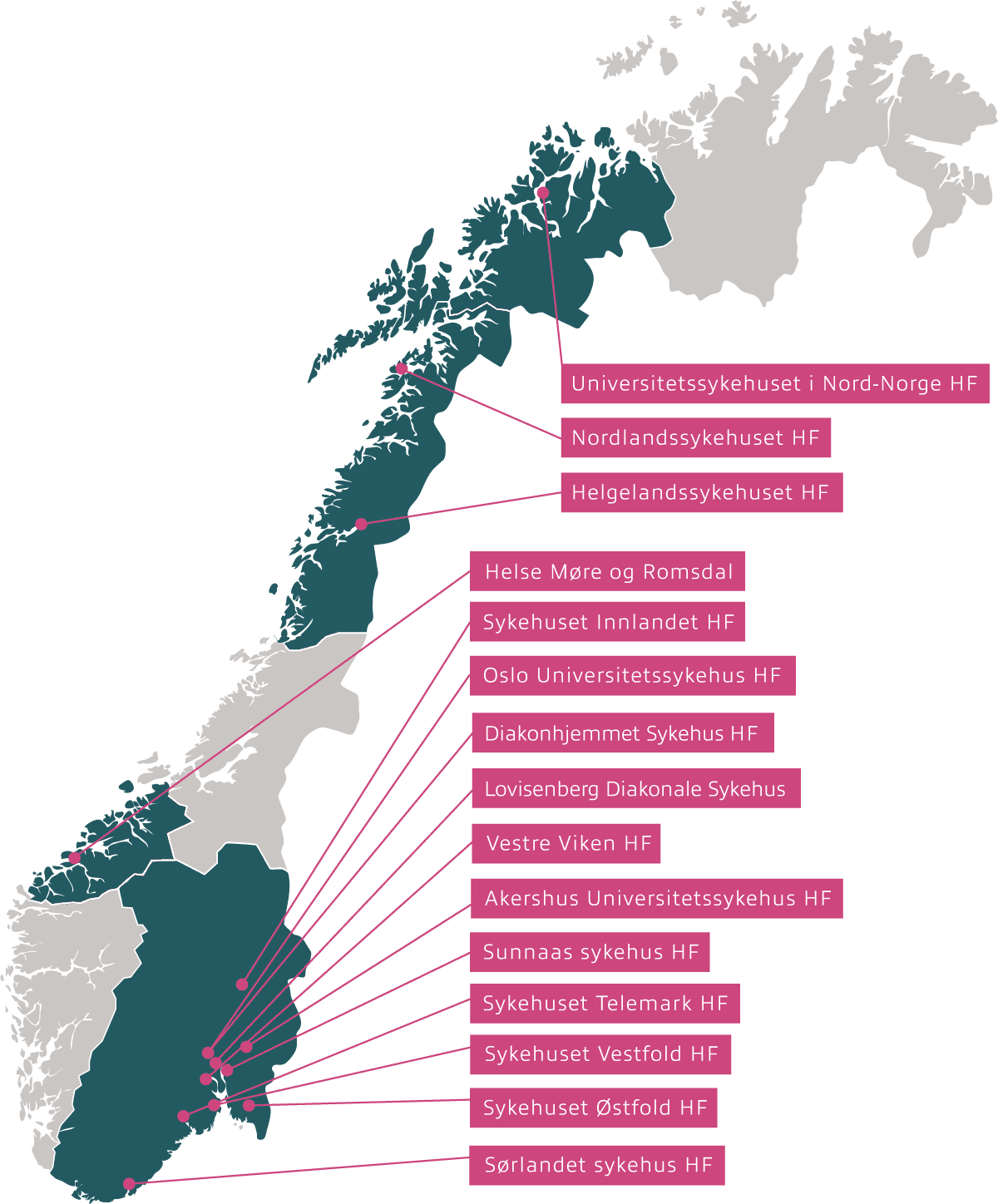
Inven2 is responsible for the following hospitals:
- Akershus University Hospital Trust
- Oslo University Hospital Trust
- Diakonhjemmet Hospital
- Lovisenberg Diaconal Hospital
- Nordland Hospital Trust
- Helgeland Hospital Trust
- Sunnaas Hospital Trust
- Vestfold Hospital Trust
- Innlandet Hospital Trust
- Telemark Hospital Trust
- Hospital of Southern Norway Trust
- Østfold Hospital Trust
- Vestre Viken Hospital Trust
- University Hospital of North Norway
- Hospital pharmacy enterprise
- Sjukehusapoteka Vest HF
- Sykehusapotekene i Midt-Norge HF
- Sykehusapotek Nord HF
- Helse Møre og Romsdal Hospital Trust
Inven2 manages the following agreement types on behalf of the health trusts:
- Clinical Trials Agreements for testing of pharmaceuticals
- Clinical Study Agreements for testing of medical devices
- Non-interventional Study Agreements, i.e. observation and register studies
- Pre-clinical studies
- Cooperation and Service Agreements
- Hospital-initiated studies with financial contribution from industry
- Agreements with all Hospital Pharmacies in Norway
Our web-based ‘Research Information Form’ must be filled and submitted as a new industry sponsored trial to Inven2 in order to start the Agreement process. Hospital staff can use the form to submit hospital-initiated trials that receive external funding.
Our role in the different stages of a clinical trial is illustrated in the processes overview for borth the start-up phase and financial follow-up phase.

