BUSINESS OPPORTUNITY
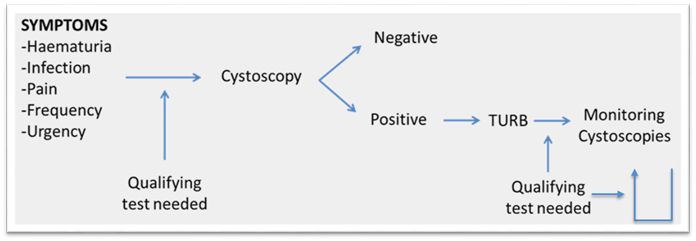
Scientists at Oslo University Hospital are developing a urine based test, addressing a major unmet need for non-invasive detection and monitoring of bladder cancer. Bladder cancer carries the highest per- lifetime, per-patient cost of any type of cancer due to high risk of recurrence necessitating frequent monitoring. Cystoscopy – the gold standard for bladder cancer detection and monitoring – is an invasive, uncomfortable and expensive intervention. Our accurate urine-based test has the potential to decrease the number of cystoscopies, sparing patients discomfort and being cost-efficient.
Inven2 AS seeks partners for co-development and/or licensing of the technology.
TECHNOLOGY
Based on a robust discovery and validation workflow, the researchers have identified eight methylome sequencing derived biomarkers for urine-based detection of bladder cancer. When analyzed in a prospective series of hematuria patients, the panel showed high performance across all tumor grades and stages; sensitivity of 92% (85% for Ta tumors), specificities of 92% and a NPV of 98%.
The test is now being further evaluated in two ongoing monitoring studies, including a Norwegian multi-center trial aiming at following 500 patients with non-muscle invasive bladder cancer over a two-year period and a trail following 59 patients at Oslo University Hospital . Preliminary data are highly promising, indicating high sensitivity of the test for early detection of recurrence
ADVANTAGE
- Highly promising data for the test to be used both to determine the likelihood of bladder cancer in patients presenting with hematuria and monitoring for bladder cancer recurrence
- A proprietary droplet digital PCR analysis platform provides highly standardized and accurate methodology.
- The test has a strong earning potential and would provide health economic benefits
IPR
Our product is expected to comprise methylated markers from two biomarker panels. The first panel of biomarkers are protected by a granted US and EP patent. The second panel is protected by pending US and EP patent applications. Patent applications related to analysis algorithm and optimized internal controls for robust digital droplet PCR analysis are pending in US.

